Pterostilbene is closely related to the Resveratrol, a well-known polyphenol stibenoid compound derived originally from the skin of red grapes. It is thought to be a powerful antioxidant that can protect the body against free radical damage that can lead to heart disease and cancer. Resveratrol has been studied in animals, and when given in extremely large daily doses to rodents has produced a number of useful health benefits, including an extended lifespan. The extremely high dose of resveratrol necessary to produce effects in animals suggests its practical use in humans might be limited. This is one of the reason why pterostilbene looks far more interesting :
Pterostilbene and Resveratrol : Structural Differences
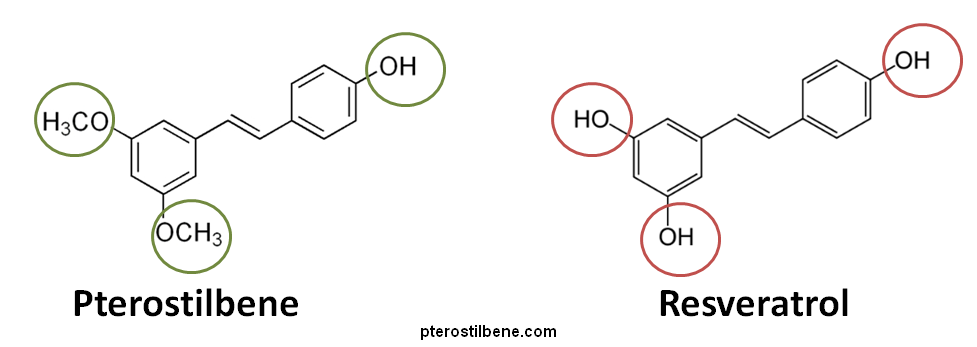
Pterostilbene is a chemical found in blueberries and grapes. It has a polyphenolic structure that is very similar to that of resveratrol. However, there are are also some important differences. Resveratrol has three hydroxy groups versus only one hydroxy group on pterostilbene. In place of the other two hydroxy groups found on resveratrol, it has two methoxy groups. This structural difference renders pterostilbene more oil-soluble and therefore much more bioavailable after oral administration than resveratrol (95% versus 20% for resveratrol) because they are more readily absorbed into the body.(1)
Bioavailability and Half-Life
When consumed orally, these stilbenoid compounds are absorbed into the body through the intestine. As they move into the blood stream, they pass through the liver. They are altered by the liver, where glucuronide or sulfates are attached to them, marking them for elimination from the body as part of the liver’s function in eliminating toxins. These tags are attached to the hydroxy groups (HO/OH) on the stilbenoid compounds. This does not happen to Pterostilbene’s methoxy molecules (H3CO/OCH3), so there is only one site that can be marked. With three hydroxy groups, resveratrol can more easily be tagged and therefore eliminated rapidly from the body. This gives Pterostilbene over 7 times more time to reach areas where it is needed via the circulatory system and be absorbed into cells.
The half-life of resveratrol is consequently only 14 minutes in the blood versus 105 minutes for pterostilbene.(2,3)

Pharmacokinetics
The difference in half-life and oral bioavailability produces a dramatic difference in the blood concentration of these two chemicals after oral consumption of the same dose. A recent study reported that these differences lead to resveratrol being rapidly cleared out of the body, while pterosilbene stays in the body and is able to accumulate in various parts of the body, where it can presumably provide protection against damaging free radicals.(1)
Studies in rats report that a 150 mg/kg oral dose of resveratrol produces a serum concentration that is almost 100-fold less than the serum concentration of a 68 mg/kg oral dose of pterostilbene shortly after administration, and by 12 hours post-dosing serum levels of resveratrol have started dropping towards zero. An oral dose of 168 mg/kg of pterostilbene maintains a very high serum level for over eight hours after administration, and doesn’t decline towards zero until almost 24 hours after administration.(1) These results clearly suggest that pterostilbene may be the preferred protective agent for practical use.
Literature Cited:
1. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats: I.M. Kapetanovic et al.; Cancer Chemother Pharmacol. (2010)
2. Inhibition of cancer growth by resveratrol is related to its low bioavailability: M. Asensi, et al.; Free Radic Biol Med. 33(3), 387 (2002)
3. Pharmacometrics of pterostilbene: preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity: C.M. Remsberg et al.; Phytother Res. 22, 169 (2008)

Leave a comment